Transesterification
Transesterification for Biodiesel Conversion
Transesterification is a chemical reaction used for the conversion of triglycerides (fats) contained in oils, (Feedstocks) into usable biodiesel. Biodiesel produced by the process of transesterification has a much lower viscosity, making it capable of replacing petroleum diesel in diesel engines.
The biodiesel production method applied worldwide at industrial level is the alkoolysis (transesterification) of triglycerides which are the main component of vegetable oils and animal fats. The most common type of esters are methyl esters, mainly because methanol is usually the cheapest alcohol. In Brazil, however, the dominant esters produced are ethyl esters since ethanol is even cheaper than alcohol. The reaction is catalyzed by bases, acids and enzymes and can take place either at low or high temperatures. The produced methyl esters of fatty acids are characterized as biodiesel while glycerol is received as a reaction byproduct.
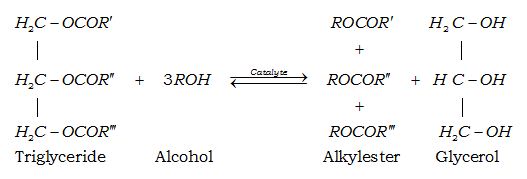
The transesterification reaction of triglycerides where R’, R’ and R’ are long chains containing carbon and hydrogen atoms (chain fatty acids).
During this reaction di-and mono-acyl-glycerol are formed as intermediates. The following diagram is qualitatively the correlation of the conversion of alkyl esters and intermediate products of the transesterification reaction time. Please note that there are differences depending on the conditions of the reaction.
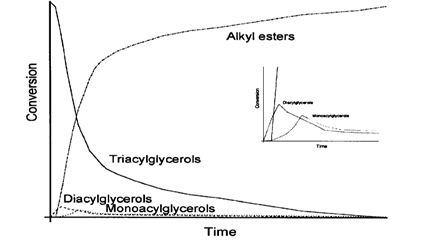
Figure 1: Qualitative diagram of the conversion of vegetable oil to methyl and intermediate products during the course of the reaction [Source: Knothe, et al (2005)]
The following figure, then, is a typical flow chart of manufacturing biodiesel, a transesterification catalyst from basic raw materials low in free fatty acids.
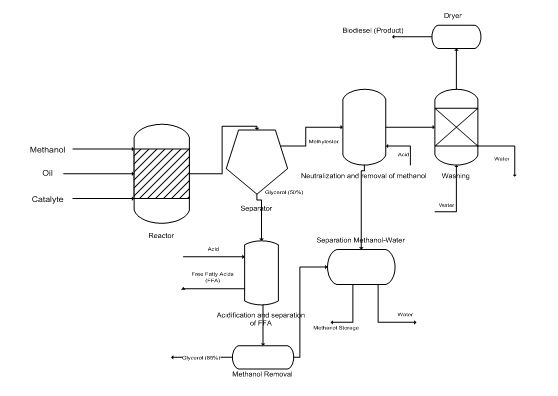
Figure 5: Flowchart for the production of biodiesel. [Source: Knothe, et al (2005) / own treatment]
Analyzing the process described in this flowchart, we should mention that the alcohol, the catalyst and the oil are mixed in a reactor and stirred for one hour at 60°C approx. Smaller capacity plants often use non-continuous reactors (batch reactors), but most large plants (those generating more than 4 million lt/year) reactors are using continuous and complete mixing. The reaction generally includes two steps: during the first one, it is added 80% alcohol and the catalyst in the oil. Then, the reactor output stream is led to the removal of glycerin, before entering the remaining 20% of alcohol.
After the reaction, the glycerol is separated from the methyl esters. Due to the low solubility of the glycerol esters, their separation can be accomplished easily and quickly using a precipitating tank or a simple centrifugation. The amount of methanol that has not reacted, acts as a solvent thereby delaying the separation of glycerol and methyl. However, excess methanol is usually not removed from the reaction stream until a complete separation of glycerol and methyl esters has been achieved, because the direct reaction of transesterification of products (higher conversion). Often, after the completion of the transesterification, water is added to the reaction mixture to facilitate the glycerol separation.
Some researchers have shown that it is possible to make oil react with methanol in the absence of a catalyst so as to minimize the need of washing with water. In this case, however, high temperatures are required and larger excess amounts of methanol. Also, there has been difficulty in replication kinetics of the reaction, which has been attributed to various catalyst on the surface of the reactor (a phenomenon which is accentuated with increasing temperature). Moreover, it has been found that at high temperature and pressure (about 90bar, 240°C, respectively) the transesterification of fats can take place without prior removal or conversion of free fatty acids. Nevertheless, most biodiesel plants for financial and safety reasons still prefer to operate at low temperatures and atmospheric pressure retaining larger reaction times.
Following the flowchart, after the glycerol separation, the mixture of the methyl esters is neutralized and then separated from impurities using vacuum distillation or evaporation, before washing with water. For the neutralization of biodiesel an acid is added, which neutralizes the residues of the basic catalyst and separates any soap residue that can be produced during the reaction. The soap reacts with the acid forming hydrophilic salts and fatty acids according to the following reaction [23]:
R-COONa + HAc ? R-COOH + NaAc Soap + fatty acid + acid ? salt
The salts are removed during washing, while the free fatty acids remain in the biodiesel. The washing water is applied to remove any remaining catalyst, soap, salt, methanol and free glycerol from biodiesel. Neutralization before washing reduces the amount of water needed and minimizes the tendency to produce emulsions. After washing, the water left in the biodiesel is distilled off under vacuum.
The stream of glycerol is removed from the separator containing glycerol 50%. It also contains some of the excess methanol and most of the catalyst and soap. In this form, the glycerol has little value and its commercial potential is small. The existence of these impurities implies that glycerol must be treated as a hazardous waste. The first step to increase the purity of glycerol is the refinement where acid is introduced in the waste stream to convert soap into free fatty acids and salts. Free fatty acids are not soluble in glycerol and separated at the top of the mixture (upper layer) which can be removed and recycled. These fatty acids can be esterified and thus used as a biodiesel feedstock in the input stream of transesterification reaction. Even then, salts are still present within glycerol. When potassium hydroxide is used as a catalyst and phosphoric acid as a reagent for the neutralization reaction, then phosphates are produced and these can be used as fertilizer. After acidification and removal of free fatty acids, methanol impurities are also removed via an extraction process through vacuum or through another type evaporator. After that glycerol produced has an 85% approximate purity, which allows it to be sold in a glycerol refining plant. There, by further distillation under vacuum or an ion exchange process, its purity can be increased from 99.5 to 99.7%.
Methanol, which is removed from the methyl ester and glycerol streams will “collect” the water produced during the process, since it is fully water miscible. This methanol-water stream should be directed to a distillation column before methanol is led back into the process. This stage is more difficult if the alcohol used is ethanol or isopropanol, due to the azeotropic mixture which is created with water. In this case a molecular sieve is required so as to remove water.
Both of SRS’ Transesterification Processes convert ester oils to biodiesel efficiently.
- Allowing feedstock flexibility.
- Allows producers to mix high FFA feedstocks with lower FFA feedstocks.
- Produces ASTM/EN Standard Fuel
- Achieves 100% conversion of Mono, Di, and Tri-Glycerides
Call one of our biodiesel specialist today to find out how to make your plant multi-feedstock capable (800)497-5841
